Stanley M Hileman, PhD
Contact Information
- Phone
- 304-293-1502
- Address
-
PO Box 9229
108 Biomedical Road
BMRC 111
Morgantown, WV 26506
Research Interests
The goal of my research is to define the neurobiological pathways controlling food intake in the female and how those pathways are integrated into a system whereby nutrition can influence fertility. To accomplish this goal, several surgical, endocrine and molecular biology techniques are employed, including radioimmunoassay, in situ hybridization histochemistry, immunocytochemistry, neuroanatomical tract tracing and RT-PCR, with both rodents and sheep being used as models. The work is focused on the neural mechanisms whereby certain circulating metabolic signals, such as leptin, insulin and IGF-1 may mediate nutrition-induced changes in reproduction as well as examining potential sex-dependent differences in these systems.
Impact of Nutrition on Reproduction

Nutrition is the major factor impacting reproduction in mammalian species. Nonetheless, little is known about the neural pathways through which inadequate nutrition reduces fertility. Our work focuses on identifying hypothalamic pathways through which endocrine signals such as leptin, insulin, or IGF-1 may influence secretion of gonadotropin releasing-hormone, a hypothalamic decapeptide essential for reproduction. Work is also underway to examine the mechanisms whereby the ability of estradiol to inhibit gonadotropin releasing-hormone is enhanced during negative energy balance. Our hope is to define the neural pathways involved in regulating reproduction during undernutrition with the aim of enhancing reproductive efficiency in domestic animals and fertility in humans.
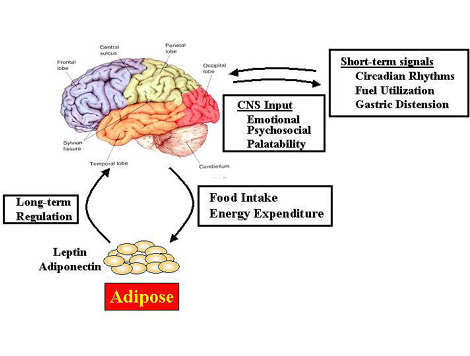
Obesity in Females
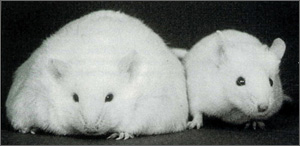
The incidence of obesity has reached epidemic proportions in the United States, and in particular, West Virginia. According to recent NIH statistics, the cost of obesity in the U.S. rose above 100 billion dollars last year. Not surprisingly, in recent years there has been an increased interest in defining the neural mechanisms whereby the brain controls food intake. However, the vast majority of these studies have examined body weight regulation in males, despite the fact that obesity in human females is at least as prevalent as in males. One focus of our laboratory is to define the neural pathways through which body weight is regulated in females. This includes the identification of pathways in the hypothalamus regulating food intake or energy expenditure that may differ between males and females and examining the mechanisms that make them different. This involves the use of several experimental paradigms, such as food restriction, high-fat feeding, and administration of leptin, an adipose-derived hormone critical in controlling body weight. We have also recently begun studies to examine adiponectin, another fat-derived hormone that may be involved in regulating body weight through actions in the brain. Previous work suggests that males and females regulate body weight differently. Thus, this work may be important for defining sex-dependent treatments for obesity in the future.
Publications
2015
· Huang J, Lin YC, Hileman S, Martin KH, Hull R, Yu HG. PP2 prevents isoproterenol stimulation of cardiac pacemaker activity. J Cardiovasc Pharmacol 65:193-202, 2015. PMID: 25658311
· Foskolos A, Ehrhardt RA, Hileman SM, Gertler A, Boisclair YR. Insensitivity of well-conditioned mature sheep to central administration of a leptin receptor antagonist. Animal 29:1-7, 2015. PMID: 26220331
· Lin Y-C, Huang J, Hileman S, Martin K, Hull R, Davis M, Yu H-G. Leptin decreased heart rate associated with increased ventricular repolarization via its receptor. American Journal of Physiology – Heart and Circulatory Physiology 309:H1731-H1739, 2015. PMID: 26408544
· Foster DL, Hileman SM. Puberty in sheep. Knobil and Neill’s Physiology of Reproduction. Tony Plant and Tony Zeleznik, eds. 2015.
2016
· Grachev P*, Porter KL*, McCosh RB, Connors JM, Hileman SM, Lehman MN, Goodman RL. Surge-like LH secretion induced by retrochiasmatic area NK3R activation is mediated primarily by ARC kisspeptin neurons in the ewe. Journal of Neuroendocrinology Vol 28 DOI: 10.1111/jne.12393, 2016. * Indicates shared first authorship. PMID: 27059932
· Lopez JA. Bedenbaugh MN, McCosh RB, Meadows LJ, Wisman B, Goodman RL, Hileman SM. Evidence that dynorphin plays an important role in puberty of female sheep. 2016, Journal of Neuroendocrinology, Vol 28, Issue 12 (December) DOI: 10.1111/jne.12445. PMID:28328155.
2017
· Thorson JF, Heidorn NL, Ryu V, Czaja K, Nonneman D, Barb CR, Hausman GJ, Prezotto LD, McCosh RB, Wright EC, White BR, Freking BA, Oliver WT, Hileman SM, Lents CA. Relationship of Neuropeptide FF receptors with pubertal maturation of gilts. Biology of Reproduction, 96:717-634, 2017. PMID:28339619
· McCosh RB, Szeligo BM, Bedenbaugh MN, Lopez JA, Hardy SL, Hileman SM, Lehman MN, Goodman RL. Evidence that endogenous somatostatin inhibits episodic, but not surge, secretion of LH in female sheep. Endocrinology. 2017 Apr 3. doi: 10.1210/en.2017-00075. [Epub ahead of print]. PMID:28379327.
· Bedenbaugh MNB, O’Connell R, Lopez JA, McCosh RB, Goodman RL, Hileman SM. Kisspeptin, GnRH and ERα colocalise with nNOS neurones in prepubertal female sheep. Journal of Neuroendocrinology NOV 25 doi: 10.1111/jne.12560. PMID:29178496
· Bedenbaugh MN, D’Oliveira M, Cardoso RC, Hileman SM, Williams GL, Amstalden M. Pubertal escape from estradiol negative feedback in ewe lambs is not accounted for by decreased ESR1 mRNA or protein in kisspeptin neurons. Endocrinology 159:426-438, 2018. PMID:29145598
2018
· Brooks SD, Hileman SM, Chantler P, Milde S, Lemaster K, Frisbee SJ, Shoemaker K, Jackson D, Frisbee J. Protection from chronic stress- and depressive symptom-induced vascular endothelial dysfunction in female rats is abolished by preexisting metabolic disease. Am J Physiol Heart Circ Physiol. 314:H1085-H1097, 2018. PMID 29451819
· Brooks SD, Hileman SM, Chantler P, Milde S, Lemaster K, Frisbee SJ, Shoemaker K, Jackson D, Frisbee J. Brooks SD, Hileman SM, Chantler P, Milde S, Lemaster K, Frisbee SJ, Shoemaker K, Jackson D, Frisbee J. Protection from vascular dysfunction in female rats with chronic stress and depressive symptoms. Am J Physiol Heart Circ Physiol. 314:H1070-H1084, 2018. PMID:29451821
· Prezotto LD, Thorson JF, Borowicz PP, Bejertness JL, Bedendbaugh MN, Hileman SM, Lents CA, Caton JS, Swanson KC. Influences of maternal nutrition on offspring visceral metabolism and hypothalamic circuitry. Dom Anim Endocrinology 65:71-79, 2018. PMID:30007131
· Bedenbaugh MN, McCosh RB, Lopez JA, Connors JM, Goodman RL, Hileman SM. A Neuroanatomical relationship of neuronal nitric oxide synthase to gonadotrophin-releasing hormone and kisspeptin neurons in adult female sheep and primates. Neuroendocrinology Jun 21. doi: 10.1159/000491393, 2018. PMID:29929191
· Weems PW, Coolen LM, Hileman SM, Hardy S, McCosh RB, Goodman RL, Lehman MN. Evidence that dynorphin acts upon KNDy and GnRH neurons during GnRH pulse termination in the ewe. Endocrinology 159:3187-3199, 2018. PMID:30016419
· Nestor CC, Bedenbaugh MN, Hileman SM, Coolen LM, Lehman MN, Goodman RL. Regulation of GnRH pulsatility in ewes. Reproduction 156:R83-R99, 2018. PMID 29880718
2019
· Goodman RL, He W, Lopez JA, Bedenbaugh MN, McCosh RB, Bowdridge EC, Coolen LM, Lehman MN, Hileman SM. Evidence that the LH surge in ewes involves both neurokinin B-dependent and –independent actions of kisspeptin. Endocrinology 2019 Oct 10. pii: en.2019-00597. doi: 10.1210/en.2019-00597. PMID:31599937
2020
· McCosh RB, Lopez JA, Szeligo BM, Bedenbaugh MN, Hileman SM, Coolen LM, Lehman MN, Goodman RL. Evidence that nitric oxide is critical for LH surge generation in female sheep. Endocrinology. 2020 Mar 1;161(3):bqaa010. doi: 10.1210/endocr/bqaa010. PMID:32067028
· Bedenbaugh MN, Bowdridge EC, Hileman SM. Role of Neurokinin B in ovine puberty. Domest Anim Endocrinol. 2020 Jan 28:106442. doi: 10.1016/j.domaniend.2020.106442. PMID: 32209283
· Lents CA, Lindo AN, Hileman SM, Nonneman DJ. Physiological and Genomic Insight into neuroendocrine regulation of puberty in gilts. Domest Anim Endocrinol. 2020 Feb 11:106446. doi: 10.1016/j.domaniend.2020.106446. PMID: 32199704
2021
· Lopez JA, Bowdridge EC, McCosh RB, Bedenbaugh MN, Lindo AN, Metzger M, Haller M, Lehman MN, Hileman SM, Goodman RL. Morphological and functional evidence for sexual dimorphism in neurokinin B signaling in the retrochiasmatic area of sheep. Accepted for publication in the Journal of Neuroendocrinology
· Porter DT, Goodman RL, Hileman SM, Lehman MN. Evidence that synaptic plasticity of glutamatergic inputs onto KNDy neurons during the ovine follicular phase is dependent on increasing levels of estradiol. Accepted for publication in the Journal of Neuroendocrinology.
· Hileman SM, Lehman MN, Coolen LM, Goodman RL. The choreography of puberty: evidence from sheep and other agriculturally important species. Accepted for Current Opinions in Endocrine and Metabolic Research.
View More Publications