Jason D. Huber, PhD
Contact Information
- Phone
- 304-293-1474
- Address
-
PO Box 9530
Department of Basic Pharmaceutical Sciences
West Virginia University
Morgantown, WV 26506
Research Interests
Under normal physiological conditions, the blood-brain barrier (BBB) is a physical and metabolic partition between the systemic circulation and the microenvironment of the brain, which serves to establish and maintain a highly regulated environment necessary for optimal neuronal function. The BBB is situated at the level of the cerebral microvasculature and is characterized by a lack of pinocytotic activity and the presence of "epithelial-like" tight junctions that allow the endothelium to closely regulate the passage of solutes into and out of the brain. In addition, the BBB has a number of channels and transporters that regulate the passage of nutrients into and wastes and toxins out of the brain. However, these same attributes that are necessary for proper neuronal function create a formidable obstacle for the entry of pharmaceutical agents into the brain; therefore, many CNS-associated pathologies (neurodegenerative diseases, multiple sclerosis, and stroke) are under-treated or not treated at all. My research focuses on two facets of BBB research: improving drug delivery to the CNS and characterizing the functional and structural integrity of the BBB in health and disease.
Effect of age on BBB alterations following MCAO and reperfusion
Age is a paramount risk factor for ischemic stroke. The impact of age and its relationship to the development of ischemic injury is an understudied, yet critical, area for investigation that serves the missions of NINDS and NIA by gaining a better understanding of stroke pathophysiology and the opportunity for improved therapeutics. The long term goal for this proposal is to improve the outcome of patients whom have suffered a stroke. As a necessary first step, we will obtain a functional and structural characterization of the blood-brain barrier (BBB) following an ischemic episode in aged rats as a means to assessing and developing therapeutic options that can be effective for people as well as laboratory animals. The rationale for the proposed research is that current animal stroke models do not adequately address age-related changes in the BBB; thus, hindering the ability to translate promising stroke therapies from the lab to the clinic. The objective of this application is to determine the impact age has on functional and structural changes occurring at the BBB following middle cerebral artery occlusion (MCAO) and reperfusion with tissue plasminogen activator (tPA). Our central hypothesis states that age is a significant risk factor in ischemic stroke that leads to increased perturbations in BBB function, characterized by increased permeability and alterations in endothelial cell-cell contacts. We propose three specific aims to test our hypothesis: 1. Investigate relative size and regional localization of blood-brain barrier functional changes following MCAO and tPA reperfusion in aged rats using in situ brain perfusion and confocal microscopy. 2. Identify BBB tight junction regulatory proteins [occludin and zonula occludens-1 (ZO-1)] following MCAO and tPA reperfusion using electrophorectic techniques and confocal microscopy. 3. Evaluate the effect of pharmacological modulation of BBB during MCAO and tPA reperfusion in aged rats using in situ brain perfusion. We expect that these findings will clearly show that age has profound effects on BBB function and structure that must be taken into account when developing and screening therapeutics to reduce stroke morbidity and mortality. Stroke is a disease of the elderly. Using an animal stroke model that takes age into account will have an impact in our gaining a better understanding how stroke affects the brain and provide a clinically relevant model for assessing potential neuroprotectants.
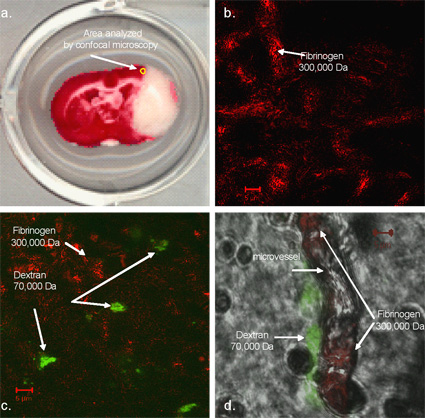
Figure depicts a TTC stained brain slice from an aged rat following MCAO and reperfusion. The yellow circle (A) denotes the area assessed by confocal microscopy. (B) This is an confocal (100x) image of fibrinogen (red) and dextrin (green) flourescent reactivity in aged rats (18 months) that underwent a (B) sham MCAO and tPA reperfusion or (C) underwent a fibrin clot MCAO and tPA reperfusion. (D) This is a confocal image (100x) with bright field that depicts the lumen of the cerebral microvessel. Within the lumen, fibrinogen reactivity (red) can be observed in the lumen of the vessel while dextran reactivity (green) is seen along the outer surface of the vessel in an aged rat (18 months) that underwent a fibrin clot MCAO and tPA reperfusion.
Effects of diabetes on blood-brain barrier function and structure
Endothelial cell dysfunction associated with microangiopathy is a primary factor in the development and progression of diabetes mellitus (DM)-related disabilities, including blindness, kidney failure, and peripheral neuropathy. While much is known about DM-induced microvascular alterations of the kidney and retina, the role DM plays in cerebral microvascular pathogenesis is understudied. Recent clinical evidence suggests DM-induced changes in the blood-brain barrier (BBB) lead to increased incidences of vascular dementia, ventricular hypertrophy, lacunar infarcts, hemorrhage, and may be a predisposing factor for Alzheimer's disease.
Our long term goal is to obtain a functional and structural characterization of the cerebral microvasculature during DM, in order to provide insight into better, safer approaches to improving the clinical outcome of DM. The objective of this application is to determine the impact DM has on the functional and structural changes occurring at the BBB during DM. The rationale for this study is that complications of DM are associated with alterations in microvascular function; however, the BBB remains largely unstudied. Preliminary data suggest that BBB function and structure are altered during DM and characterized by increased BBB permeability, regions of greater susceptibility, and alterations in the interactions of the tight junction regulatory proteins occludin and zonulae occludens-1 (ZO-1). Our central hypothesis states that DM leads to progressive deterioration of BBB integrity, characterized by increased permeability and alterations in endothelial cell-cell contacts.
We plan to test our central hypothesis and accomplish the objective of this application by pursuing the following two specific aims:
Specific Aim 1: Investigate relative size and regional localization of BBB paracellular changes during DM. The working hypothesis is that DM induces a progressive deterioration in BBB function, characterized by increased paracellular permeability and a greater number of brain areas affected as compared to age-matched controls.
Specific Aim 2: Identify changes in tight junction regulatory proteins (ZO-1 and occludin) during DM. The working hypothesis is that DM induces an increased deterioration of BBB cell-cell contacts, characterized by changes in ZO-1 and/or occludin expression and localization as compared to age-matched controls.
The proposed work is innovative because it addresses the significance of BBB functional and structural deterioration during the onset and progression of DM. It is our expectation that the results of this application will clearly show that DM has profound effects on BBB function and structure that must be taken into account when assessing the pathophysiology of long term DM-induced complications. These results will be significant because they will serve as the foundation for a programmatic effort to better assess CNS complications in diabetic patients and serve as the basis for future NIH funding.
Major complications associated with DM are manifest in damage to the vascular system, leading to decreased quality of life for those who suffer from the disease. Due to the progressive nature of DM and the unique characteristics of the BBB, effects of DM on the cerebromicrovasculature are, in many ways, different from other microvascular beds and barrier systems, such as seen at the retina and peripheral nerves. Often the adverse effects seen at the BBB can be more insidious as vascular dysregulation is less perceptible at first and by the time clinical signs are noticeable, neurological damage has occurred. Understanding how DM progressively alters BBB function and structure is an under investigated yet important area of DM-related research. We expect this proposal is a start toward addressing this need and our lab is uniquely qualified to carry out this research.
Publications
[2017]
- Logsdon AF, Lucke-Wold BP, Turner RC, Li X, Adkins CE, Mohammad AS, Huber JD, Rosen CL, Lockman PR (2017). A mouse model of focal vascular injury induces astrocyte reactivity, tau oligomers, and aberrant behavior. Arch Neurosci, 4(2). pii: e44254. PMCID: PMC5529099
- Lucke-Wold BP, Logsdon AF, Turner RC, Huber JD, Rosen CL (2017). Endoplasmic reticulum stress modulation as a target for ameliorating effects of blast induced traumatic brain injury. J Neurotrauma, 34(S1):S62-S70.0
- Turner RC, Naser ZJ, Lucke-Wold BP, Logsdon AF, Vangilder RL, Matsumoto RR, Huber JD, Rosen CL(2017). Single low-does lipopolysaccharide preconditioning: neuroprotective against axonal injury and modulates gilal cells. Neuroimmunol Neuroinflamm, 4:6-15. PMCID: PMC5289820
- Lucke-Wold BP, Phillips M, Turner RC, Logsdon AF, Smith KE, Huber JD, Rosen CL, Regele JD (2017). Elucidating the role of compression waves and impact duration for generating mild traumatic brain injury in rats. Brain Inj, 31(1):98-105. PMCID: PMC5247354
[2016]
- Turner RC, DiPasquale K, Logsdon AF, Tan Z, Naser ZJ, Huber JD, Rosen CL, Lucke-Wold BP (2016). Behavioral and biochemical effects of ketamine and dextromethorphan relative to its antidepressant-like effects in Swiss Webster mice. Neuroreport, 27(14): 1004-11. PMCID: PMC5020901
- Logsdon AF, Lucke-Wold BP, Nguyen L, Matsumoto RR, Turner RC, Rosen CL, Huber JD (2016). Salubrinal reduces oxidative stress, neuroinflammation and impulsive-like behavior in a rodent model of traumatic brain injury. Brain Res, 1643:140-51. PMCID: PMC5578618
- Turner RC, DiPasquale K, Logsdon AF, Tan Z, Naser ZJ, Huber JD, Rosen CL, Lucke-Wold BP (2016). The role for infarct volume as a surrogate measure of functional outcome following ischemic stroke. J Syst Integr Neurosci, 2(4). PMCID: PMC5347398
- Lucke-Wold BP, Logsdon AF, Manoranjan B, Turner RC, McConnell E, Vates GE, Huber JD, Rosen CL, Simard JM (2016). Aneurysmal subarachnoid hemorrhage and neuroinflammation: A comprehensive review. Int J Mol Sci, 17(4):497. PMCID: PMC4848953
[2015]
- Turner RC, Lucke-Wold BP, Logsdon AF, Robson MJ, Lee JM, Bailes JE, Dashnaw ML, Huber JD, Petraglia AL, Rosen CL. Modeling Chronic Traumatic Encephalopathy: The Way Forward for Future Discovery. Front Neurol (2015); 6:223. doi: 10.3389/fneur.2015.00223. eCollection 2015. Review. PMID: 26579067
- Turner RC, Lucke-Wold BP, Logsdon AF, Robson MJ, Dashnaw ML, Huang JH, Smith KE, Huber JD, Rosen CL, Petraglia AL. The Quest to Model Chronic Traumatic Encephalopathy: A Multiple Model and Injury Paradigm Experience.Front Neurol (2015); 6:222. doi: 10.3389/fneur.2015.00222. eCollection 2015. PMID: 26539159
- Lucke-Wold BP, Nguyen L, Turner RC, Logsdon AF, Chen YW, Smith KE, Huber JD, Matsumoto R, Rosen CL, Tucker ES, Richter E. Traumatic brain injury and epilepsy: Underlying mechanisms leading to seizure. Seizure (2015); 33:13-23. doi: 10.1016/j.seizure.2015.10.002. Epub 2015 Oct 29. Review. PMID: 26519659
- Lucke-Wold BP, Naser ZJ, Logsdon AF, Turner RC, Smith KE, Robson MJ, Bailes JE, Lee JM, Rosen CL, Huber JD. Amelioration of nicotinamide adenine dinucleotide phosphate-oxidase mediated stress reduces cell death after blast-induced traumatic brain injury. Transl Res (2015); 166(6):509-528.e1. doi: 10.1016/j.trsl.2015.08.005. Epub 2015 Sep 8. PMID: 26414010
- Lucke-Wold BP, Turner RC, Logsdon AF, Nguyen L, Bailes JE, Lee JM, Robson MJ, Omalu BI, Huber JD, Rosen CL. Endoplasmic reticulum stress implicated in chronic traumatic encephalopathy. J Neurosurg (2015); 18:1-16. PMID: 26381255
- Tan Z, Lucke-Wold BP, Logsdon AF, Turner RC, Tan C, Li X, Hongpaison J, Alkon DL, Simpkins JW, Rosen CL, Huber JD. Bryostatin extends tPA time window to 6 h following middle cerebral artery occlusion in aged female rats.Eur J Pharmacol (2015); 764:404-12. doi: 10.1016/j.ejphar.2015.07.035. PMID: 26189021
- Logsdon AF, Lucke-Wold BP, Turner RC, Huber JD, Rosen CL, Simpkins JW.Role of Microvascular Disruption in Brain Damage from Traumatic Brain Injury.Compr Physiol (2015); 5(3):1147-60. doi: 10.1002/cphy.c140057. PMID: 26140712
- Lucke-Wold BP, Smith KE, Nguyen L, Turner RC, Logsdon AF, Jackson GJ, Huber JD, Rosen CL, Miller DB. Sleep disruption and the sequelae associated with traumatic brain injury. Neurosci Biobehav Rev (2015); 55:68-77. doi: 10.1016/j.neubiorev.2015.04.010. Review. PMID: 25956251
[2014]
- Logsdon AF, Turner RC, Lucke-Wold BP, Robson MJ, Naser ZJ, Smith KE, Matsumoto RR, Huber JD, Rosen CL. Altering endoplasmic reticulum stress in a model of blast-induced traumatic brain injury controls cellular fate and ameliorates neuropsychiatric symptoms. Front Cell Neurosci (2014); 8:421. doi: 10.3389/fncel.2014.00421. eCollection 2014. PMID: 25540611
- Lucke-Wold BP, Logsdon AF, Turner RC, Rosen CL, Huber JD. Aging, the metabolic syndrome, and ischemic stroke: redefining the approach for studying the blood-brain barrier in a complex neurological disease. Adv Pharmacol(2014); 71:411-49. doi: 10.1016/bs.apha.2014.07.001. Review. PMID: 25307225