Bernard G Schreurs, PhD
Contact Information
- Phone
- 304-293-0497
- Address
-
PO Box 9303
8 Medical Center Drive
1024 RNI Building
Morgantown, WV 26506
Research Interests
My research is in learning and memory, synaptic plasticity, and preclinical models of Alzheimer's disease and post traumatic stress disorder. The lab has been studying the neural basis of learning and memory using a number of model systems including the marine snail, Hermissenda, the rabbit eyelid/nictitating membrane preparation, and human eyelid conditioning. Studies have focused on revealing the behavioral laws and identifying the neural substrates of learning and memory as well as examining the synaptic plasticity that occurs at these substrates. We use classical conditioning as a behavioral paradigm and slice electrophysiology as a way of recording the changes that learning induces in the brain. We are currently using patch clamp recording to identify specific ion channels involved in learning-dependent changes in the cerebellum. We have developed an animal model of Alzheimer's disease which can produce Alzheimer's-like pathology using simple dietary manipulations. We also have an animal model of some aspects of post traumatic stress disorder that has yielded insights into possible behavioral treatments for the disorder.
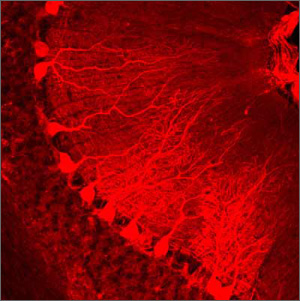
Immunohistochemical staining of Purkinje cells in a rabbit cerebellar cortex
Cholesterol Effects on Learning and Memory
Despite the crucial role played by cholesterol and copper in nutrition and normal brain function, recent evidence indicates that they may both be important factors in the etiology of Alzheimer's disease. We have provided evidence for the role of cholesterol and copper in Alzheimer's disease by showing that the addition of trace amounts of copper [0.12 PPM] to the water given cholesterol-fed rabbits can induce beta-amyloid accumulation including senile plaque-like structures in the hippocampus and temporal lobe and can significantly retard the ability of rabbits to learn a difficult trace conditioning task. The beta amyloid deposits do not affect the ability of rabbits to detect or respond to the training stimuli nor to learn a simpler delay conditioning task. Trace amounts of copper in drinking water may influence clearance of beta amyloid from the brain at the level of the interface between the blood and cerebrovasculature and combined with high cholesterol may be a key component to the accumulation of beta amyloid in the brain which, in turn, has a significant impact on learning and memory. Cholesterol-fed rabbits have at least 12 pathological markers seen in Alzheimer's disease suggesting that the cholesterol-fed rabbit is a good animal model for studying Alzheimer's disease.
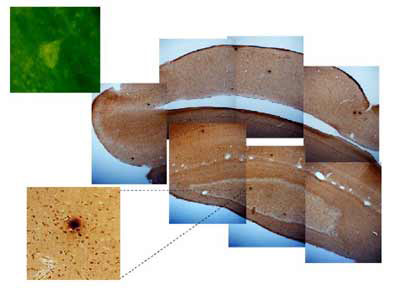
Cholesterol and copper in the diet combine to induce beta-amyloid plague-like structures in the rabbit brain.
Reflex Modification: a Model of Post-traumatic Stress Disorder
We originally coined the term conditioning-specific reflex modification (CRM) to describe changes in the rabbit's nictitating membrane response (NMR) to an unconditioned stimulus (US) when the US was tested by itself after classical conditioning. Following pairings of a tone conditioned stimulus (CS) and electrodermal stimulation, NMRs to the US increased in size and peaked later, especially at intensities weaker than the training intensity. Post-traumatic stress disorder [PTSD] is a psychological disorder resulting from exposure to a traumatic event. The symptoms associated with PTSD include persistent re-experiencing of the traumatic event, persistent avoidance of stimuli associated with the trauma, numbing of general responsiveness, and persistent symptoms of increased arousal. One of the hallmarks of PTSD is intense psychological distress and/or physiological reactivity to cues that symbolize or resemble an aspect of the traumatic event. This learning-related aspect of PTSD has some similarities to CRM. Like a Vietnam veteran who "hits the deck" when he hears a car backfire, our rabbits now blink to a mild electrical stimulus as if it were a strong stimulus. We would argue that the backfire is a weak "US" rather than a "CS." By presenting the "CS" and weak version of the "US" separately, we have been able to extinguish CRM. This may prove to be a more a clinically relevant way of reducing the heightened physiological activity associated with PTSD.
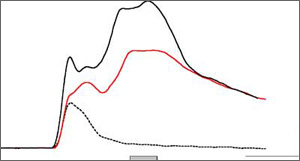
Figure shows simple reflex responses are increased in size and become more complex after learning.
Cerebellar Purkinje Cell Properties are Modified by Learning
Classical conditioning of the rabbit nictitating membrane response involves changes in both synaptic and intrinsic membrane properties of Purkinje cell dendrites in the cerebellar cortex and pharmacological evidence suggests that a 4-aminopyridine (4AP) sensitive potassium channel underlies the membrane changes. We characterized IA currents in adult, rabbit cerebellar Purkinje cells to determine whether IA might be the target channel involved in learning. Standard whole-cell voltage clamp of Purkinje cell somas and dendrites revealed a fast activating and inactivating current with half maximal activation at -27.08±3.48mV and -25.51±1.15mV in soma and dendrites, respectively, half maximal inactivation at -58.91±2.34 mV and -49.90±2.58mV and a recovery time constant of 22.81±1.92ms and 16.60±4.26ms. More importantly, there was an overlap of activation and incomplete inactivation at potentials from -60mV to -40mV suggesting a "window" current that was responsible for subthreshold variations of membrane potential and might underlie conditioning-specific increases in Purkinje cell excitability.
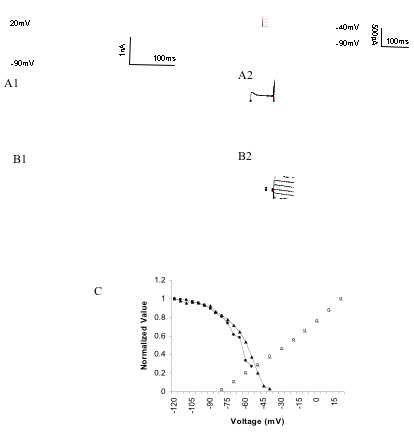
Figure shows the voltage dependence of both activation and inactivation of transient potassium currents in rabbit cerebellar Purkinje cells thought to be involved in learning and memory.
Publications
Selected Publications
[2021]
- O'Dell, D.E., Schreurs, B.G., Smith-Bell, C., Wang D. (2021). Disruption of rat deep cerebellar perineuronal net alters eyeblink conditioning and neuronal electrophysiology. Neurobiology of Learning and Memory, Neurobiol Learn Mem. 2021 Jan;177:107358 doi: 10.1016/j.nlm.2020.107358.
[2019]
- Burhans LB, Schreurs BG. (2019). Inactivation of the interpositus nucleus during unpaired extinction does not prevent extinction of conditioned eyeblink responses or conditioning-specific reflex modification. Behav Neurosci. 2019 Aug;133(4):398-413.
- Schreurs BG. (2019). Changes in cerebellar intrinsic neuronal excitability and synaptic plasticity result from eyeblink conditioning. Neurobiol Learn Mem. 2019 Dec;166:107094.
[2018]
- Wang D, Smith-Bell CA, Burhans LB, O'Dell DE, Bell RW, Schreurs BG (2018). Changes in membrane properties of rat deep cerebellar nuclear projection neurons during acquisition of eyeblink conditioning. Proc Natl Acad Sci U S A. 115(40):E9419-E9428.
- Burhans LB, Schreurs BG (2018). Inactivation of the interpositus nucleus blocks the acquisition of conditioned responses and timing changes in conditioning-specific reflex modification of the rabbit eyeblink response. Neurobiol Learn Mem. 4;155:143-156.
- Schreurs BG, Smith-Bell CA, Burhans LB (2018). Sex differences in a rabbit eyeblink conditioning model of PTSD. Neurobiol Learn Mem. pii: S1074-7427(18)30100-X.
- Burhans LB, Smith-Bell CA, Schreurs BG (2018). Propranolol produces short-term facilitation of extinction in a rabbit model of post-traumatic stress disorder. Neuropharmacology 135:386-398.
- Schreurs BG, Smith-Bell CA, Burhans LB (2018). Delayed unpaired extinction as a treatment for hyperarousal of the rabbit nictitating membrane response and its implications for treating PTSD. J Psychiatr Res 99:1-9.
[2017]
- Smith-Bell CA, Schreurs BG (2017). Grouping subjects based on conditioning criteria reveals differences in acquisition rates and in strength of conditioning-specific reflex modification. Neurobiol Learn Mem. 145:172-180.
- Burhans LB, Smith-Bell CA, Schreurs BG (2017). Effects of systemic glutamatergic manipulations on conditioned eyeblink responses and hyperarousal in a rabbit model of post-traumatic stress disorder. Behav Pharmacol, 28(7):565-577.
- Brooks SW, Dykes AC, Schreurs BG (2017). A high-cholesterol diet increases 27-hydroxycholesterol and modifies estrogen receptor expression and neurodegeneration in rabbit hippocampus. J Alzheimers Dis, 56(1):185-196.
[2016]
- Schreurs BG, Sparks DL (2016). Dietary high cholesterol and trace metals in the drinking water increase levels of ABCA1 in the rabbit hippocampus and temporal cortex. J Alzheimers Dis. 49(1):201-9.
[2015]
- Schreurs BG, Burhans LB. (2015) Eyeblink classical conditioning and post-traumatic stress disorder - a model systems approach. Front Psychiatry, 6:50.
- Denvira J, Neitch S, Fan J, Niles RM, Boskovic G, Schreurs BG, Primerano DA, Alkon DL (2015). Identification of the PS1 Thr147Ile variant in a family with very early onset dementia and expressive aphasia. Journal of Alzheimer's Disease, 46, 483-490.
- Schreurs BG. Burhans LB (2015) Eyeblink classical conditioning and post traumatic stress disorder – a model systems approach. Frontiers in Psychiatry, 6:50.
[2014]
- Wang D, Schreurs BG (2014). Maturation of membrane properties of neurons in the rat deep cerebellar nuclei. Developmental Neurobiology, 74, 1268-1276.
[2013]
- Schreurs BG, Smith-Bell CA, Wang D, Burhans LB (2013).Dietary cholesterol degrades rabbit long term memory for discrimination learning but facilitates acquisition of discrimination reversal. Neurobiology of Learning and Memory, 106, 238-245.
[2013]
- Schreurs BG, Burhans LB, Smith-Bell CA, Mrowka SW, Wang D (2013).Ontogeny of trace eyeblink conditioning to shock-shock pairings in the rat pup. Behavioral Neuroscience, 127, 114-120.
[2012]
- Gonzalez-Joekes J, Schreurs BG (2012). Anatomical characterization of a rabbit cerebellar eyeblink premotor pathway using pseudorabies and identification of a local modulatory network in anterior interpositus. Journal of Neuroscience, 32, 12472-12487.