Preclinical PET-MR Imaging
Pre-Clinical PET-MRI
Beginning with Dr. Raylman's 1991 Ph.D. (Reduction of positron range effects by the application of a magnetic field: For use with positron emission tomography) our group has a long-standing, multi-year involvement in the development of combined MRI and PET systems. The different PET-MRI systems developed at WVU are described below.
Limited-Angle MRI-PET Scanner
Our first system consisted of two opposed detector heads, operating in coincidence mode. Each MRI-PET detector module consisted of an array of LSO detector elements (20x20 arrays of 2.5x2.5x15mm3 LSO elements) coupled through a long fiber optic light guide to a single Hamamatsu flat panel position sensitive photomultipier tube (PSPMT). The use of light guides allowed the PSPMTs to be positioned outside the bore of a 3T MRI scanner where the magnetic field is relatively small. The custom MRI saddle coil was constructed by our group. To test the device, simultaneous MRI and PET images of the brain of a male Sprague Dawley rat injected with FDG were successfully obtained. The images revealed no apparent artifacts in either image set.

Pictures and images from the combined MRI and PET imaging of a rat. (A) The prototype MRI-PET insert. The tube in the center of the photo is supplying gaseous anesthesia to the rat; (B) Picture of the MRI-PET imaging insert inside the 3T MRI scanner; (C) co-registered FDG PET-MRI sagittal image of a rat head. Areas of increased FDG uptake have been labeled: a is the anterior cerebral cortex in the region where olfactory and pre-optic areas reside, b is the olfactory bulb and c indicates the position of the medulla oblongata. The label d shows the position of the spinal chord. Enhanced FDG accumulation in the cerebellum is labeled e. The areas labeled f and g are regions of extra-cranial FDG uptake.
Combined PET-MRS Data Acquisition
While the imaging aspects of MRI-PET could be substantial, the real power of MRI-PET may be the acquisition of PET images in conjunction with magnetic resonance spectroscopy (MRS) data. To explore this concept, we modified our MRI-PET system so that it was better suited to acquire MRS data. This task required replacement of the saddle RF coil used to image the rat with a twelve-leg birdcage RF coil (Nova Medical, LLC).

Picture showing the combined PET-MRS scanner insert. A 30mm-diameter hollow glass sphere was filled with seventeen substances commonly found in the human brain is shown at the center of the scanner insert. The concentrations of each chemical in the phantom were [13]: GABA 1mM, L-aspartate (Asp) 1 mM, choline (Ch) 0.9mM, creatine (Cr) 5.4mM, ethanolamine (Et) 3.3mM, glucose (Glc) 1mM, glutamate (Glu) 8.1mM, glutamine (Gln) 1.6mM, glutathione (GSH) 2mM, glycerophosphorylcholine (GPC) 1mM, myo-Inositol (mI) 3.5mM, lactate (Lac) 0.4mM, phosphocreatine (PC) 1.6 mM, phosphorylcholine (PCh) 0.6 mM, taurine (Tau) 0.9mM, N-acetyl aspartate (NAA) 7.6mM and threonine (Thr) 0.3mM. The metabolites were prepared in a buffer containing 1 mM DSS, 72mM K2HPO4, 28mM KH2PO4, 200mM Na formate, 1g/L NaN3. The pH of the mixture was adjusted to be 7.2. To produce the PET signal, 0.12µCi/ml of 18F-Fluorodeoxyglucose (FDG) was added to the sphere (total radioactivity was approximately 14.4µCi) approximately 5 minutes prior to data acquisition.
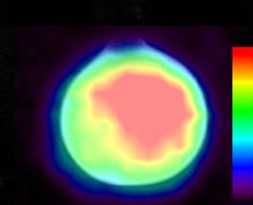
Co-registered MRI-PET image of the sphere containing the metabolites.
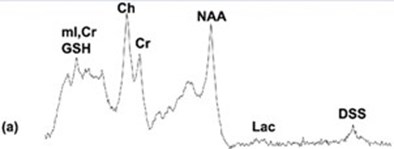
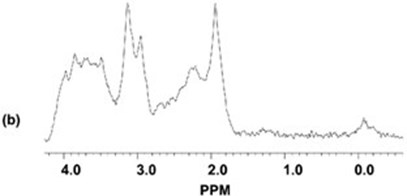
Results from the 1D-MRS measurements with and without the MR-compatible PET insert in the scanner. The top trace (a) shows the spectrum obtained with the PET scanner present, the bottom trace (b) shows the spectrum obtained without the PET scanner. The NAA/Cr metabolite ratios measured from the 1D spectra with the PET scanner in place and without the PET scanner were 1.58 and 1.52, respectively (the actual ratio present in the phantom is 1.4). Thus, there was no noticeable effect of the PET scanner insert on the MRS measurements.
PET-MRI Preclinical-Imaging Insert
Encouraged by the the success of the limited-angle PET/MRI scanner, our group has recently developed an MRI-PET insert for a Siemens 3T Verio scanner. This system consists of a ring of water-cooled, silicon photomultipliers and custom MRI RF coils. This project is a collaborative effort among West Virginia University, Nova Medical, LLC., the University of Maryland Medical School and the University of Washington.
To optimize the imaging performance of each component of the system, the PET scanner is located outside the bore of the MR scanner. Thus, there is minimal interference between the two systems; no design concessions had to be made to accommodate use of the PET scanner inside the MR scanner.
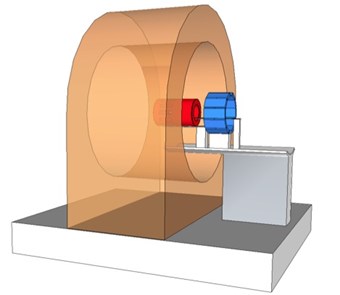
Schematic drawing of the WVU preclinical MRI-PET insert.
Although the PET scanner is not located inside the bore of the MRI scanner, there is still a relatively high magnitude magnetic field present at its position. Consequently, the standard PSPMTs had to be replaced by arrays of SiPMs (Array4p9 from SensL Photonics). Specifically, two Array4p9s were coupled through a thin light guide to a 26x58 array of LYSO detector elements (1.51.510mm^3; pitch= 1.57mm) to create a PET detector module. The performance of the SIPM was enhanced by cooling them to ~10degC by surrounding the edges of each module with a copper enclosure cooled with circulating fluid (see picture below) The presence of the copper cooling jackets in the bore of the MR scanner would normally be problematic, but since the PET scanner is not inside the MRI there are no deleterious effects on the MRI image quality. To create the scanner, twelve detector modules were formed into a ring (see picture below).
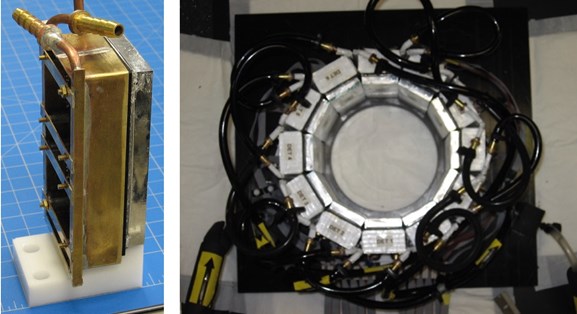
Picture of one of the MR-compatible PET detector modules (left) and picture of the PET detector ring (cooling lines are shown).
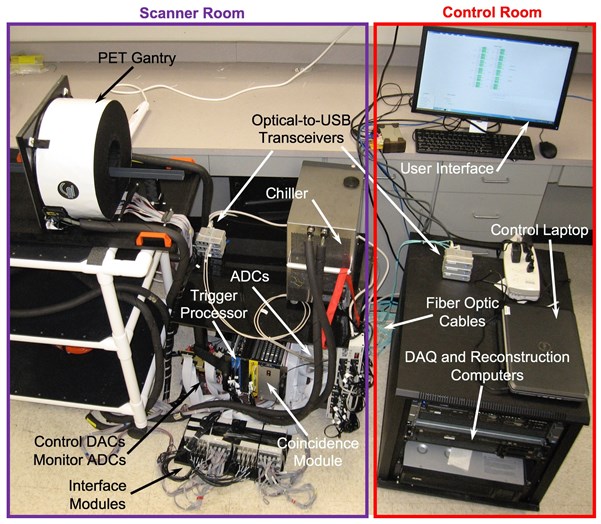
Picture showing the complete PET component of the system. Note that the elements located in the MRI scanner and control rooms are labeled.
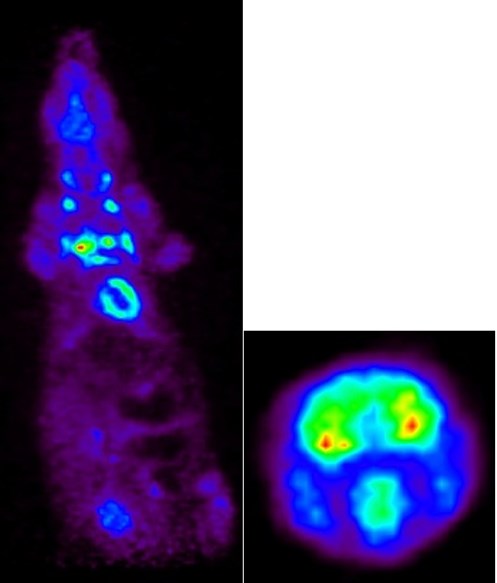
FDG-PET images of a rat: Whole body (left) and brain (right).
In addition to the PET scanner insert, two custom RF antennas (8cm and 12cm diameters) optimized for use with small animals (rats to rabbits) were constructed in collaboration with Nova Medical, LLC. These birdcage coils have transmit-receive capabilities with dual-preamplifier interfaces. A small track connects the MRI and PET components of the system so that the enclosure holding the animal can positioned in each scanner.
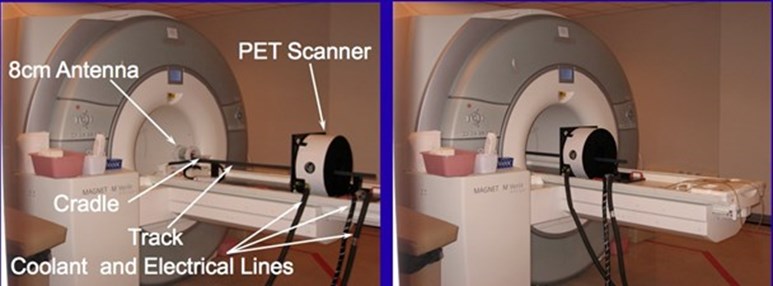
Pictures showing insert at its extended position (left) and inserted into the 3T Vereo scanner (right).
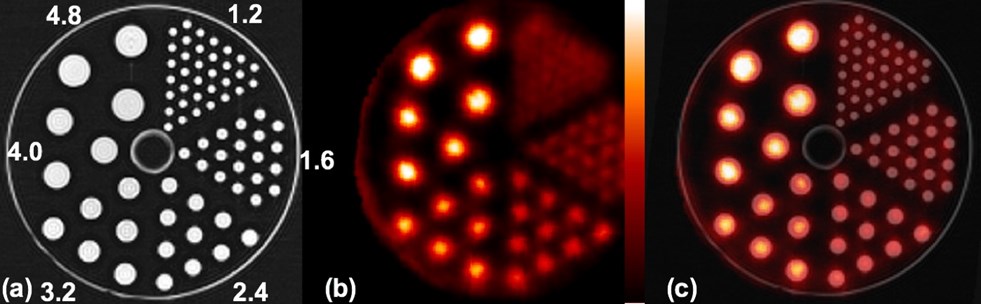
MR image of micro-hot rod phantom (the diameters of the rods are shown) (a), PET image of the phantom (b) and registered image (c).
Acknowledgements
This work was supported in part by the National Institute of Biomedical Imaging and Bioengineering (Grant Number R01 EB007349).
MRI-PET Publications from our Group
- R.R. Raylman, B.E. Hammer, N. L. Christensen. Combined MRI-PET Scanner: A Monte Carlo Evaluation of the Improvements in PET Resolution Due to the Effects of a Static Homogeneous Magnetic Field. IEEE Transactions on Nuclear Science, 1996;43:2406-2412.
- R.R. Raylman, S. Majewski, B. Kross, S. K. Lemieux, S.S. Velan, V. Popov, J. Proffitt, M.F. Smith, A.G. Weisenberger, R. Wojcik, Initial Testing of a Prototype MRI-Compatible PET Imager, Nuclear Instruments and Methods in Physics Research, Section A, 2006;569(2):306-309.
- R.R. Raylman, S. Majewski, S.K. Lemieux, S.S. Velan, B. Kross, V. Popov, M.F. Smith, A.G. Weisenberger, C. Zorn, G.D. Marano, Simultaneous MRI and PET Imaging of a Rat Brain, Physics in Medicine and Biology, 2006;51:6371‑6379.
- R.R. Raylman, S. Majewski, S.S. Velan, S.K. Lemieux, B. Kross, V. Popov, M.F. Smith, A.G. Weisenberger, Simultaneous Acquisition of magnetic Resonance Spectroscopy (MRS) Data and Positron Emission Tomography (PET) Images with a Prototype MR-Compatible, Small Animal PET Imager, Journal of Magnetic Resonance, 2007;186(2):305-310.
- A.V. Stolin, S. Majewski, R.R. Raylman, Novel Method of Temperature Stabilization for SiPM-Based Detectors, IEEE Transactions on Nuclear Science, 2013;60(5):3181-3187 (NIHMSID 477184).
- A.V. Stolin, S. Majewski, G. Jaliparthi, R.R. Raylman, J. Proffitt, Evaluation of Imaging Modules Based on SensL Array SB-8 for Nuclear Medicine Applications, IEEE Transactions on Nuclear Science, 2014;61(5):2433-2438.
- R.R. Raylman, A.V. Stolin, S. Majewski, J. Proffitt, A Large Area, Silicon Photomultiplier-Based PET Detector Module, Nuclear Instruments and Methods in Physics Research, Section A, 2014;735C:602-609 (NIHMSID 532031).
- R. R.Raylman, P. Ledden, A.V. Stolin, B. Hou, G. Jaliparthi ,P.F. Martone, Small Animal, Positron Emission Tomography-Magnetic Resonance Imaging System Based on a Clinical Magnetic Resonance Imaging Scanner: Evaluation of Basic Imaging Performance, Journal of Medical Imaging, 2018;5(3):033504.